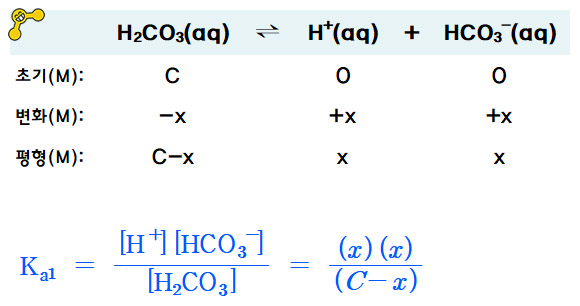Thermal stability of β-H2CO3 in the product mixture at 260 K, ∼400 mbar... | Download Scientific Diagram
SOLVED: If the carbonic acid (H2CO3) concentration in a sample of blood is 0.00125 M, determine the bicarbonate ion (HCO3-) concentration required to buffer the pH of blood at pH = 7.40.

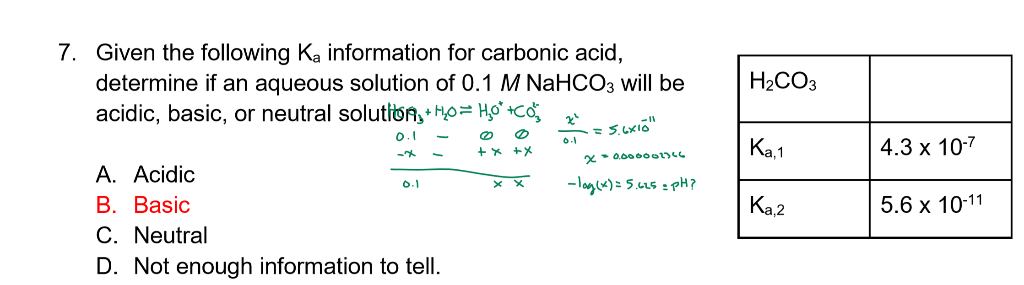
The Ka of carbonic acid is 4.3 x 10-7. H2CO3 = H+ + HCO3 This means that H2co3 is a____. A.good - Brainly.com
Find the concentration of H+, HCO^-3 and CO^2-3, in a 0.01M solution of carbonic acid if the - Sarthaks eConnect | Largest Online Education Community

Sejumlah H2CO3 (Ka = 4,3 x 1O-7) dicampurkan dengan larutan Ca(OH)2 membentuk larutan penyangga. Setelah - Brainly.co.id
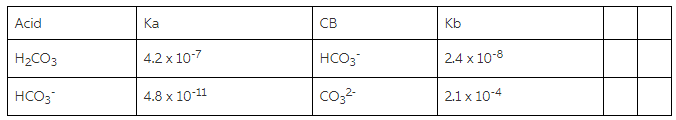
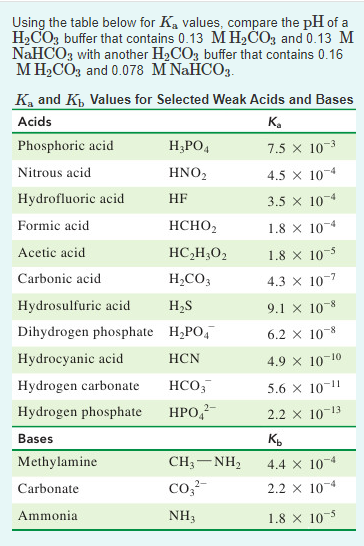
A) Using the information in the chart about Ka/Kb values, whichspecies will you use? - Home Work Help - Learn CBSE Forum

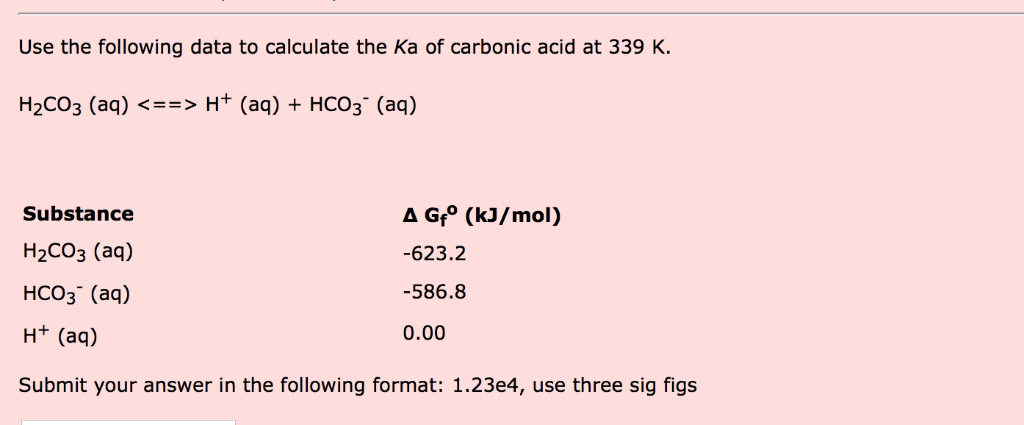
For carbonic acid the Ka1 = 4.30 × 10^-7 and the Ka2 = 5.62 × 10^-11. Calculate the pH of a 0.15 M solution of Na2CO3 :
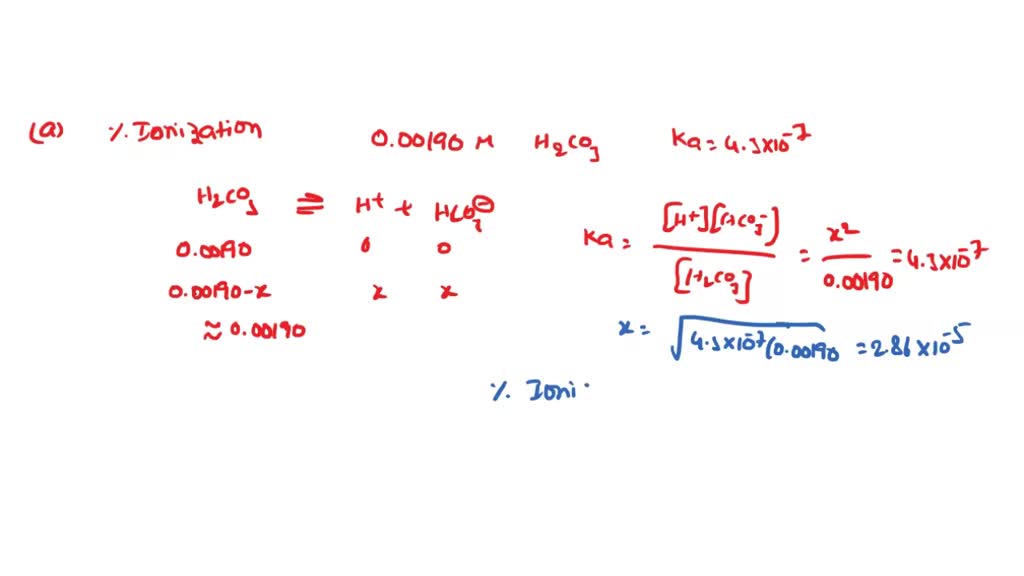
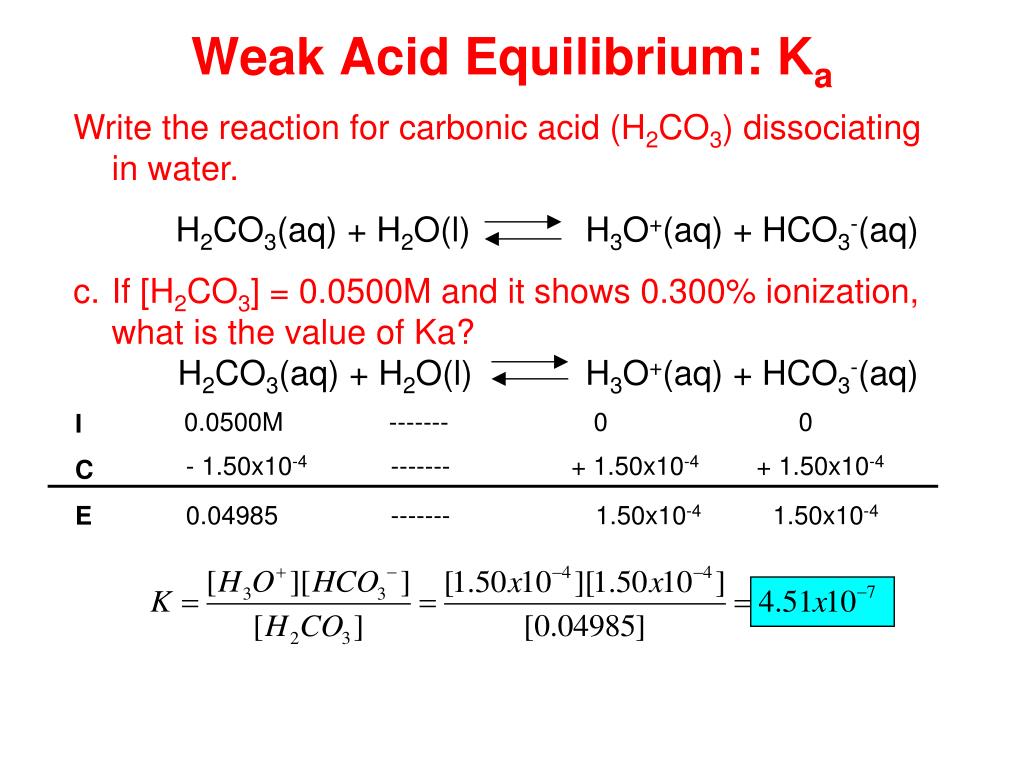
SOLVED: Calculate the percent ionization of carbonic acid (H2CO3) in solutions of each of the following concentrations (Ka = 4.3e-07.) (a) 0.281 M % (b) 0.366 M % (c) 0.641 M %
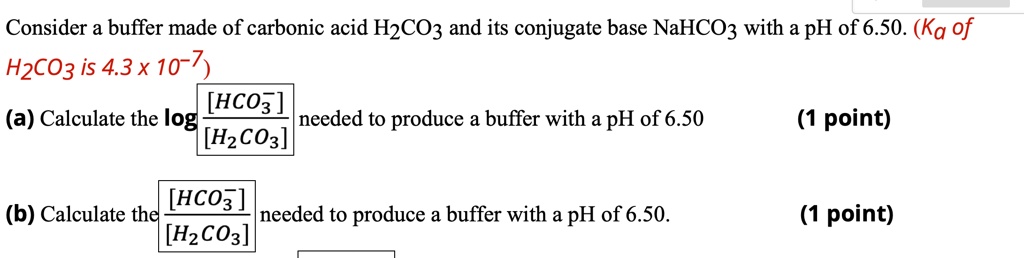
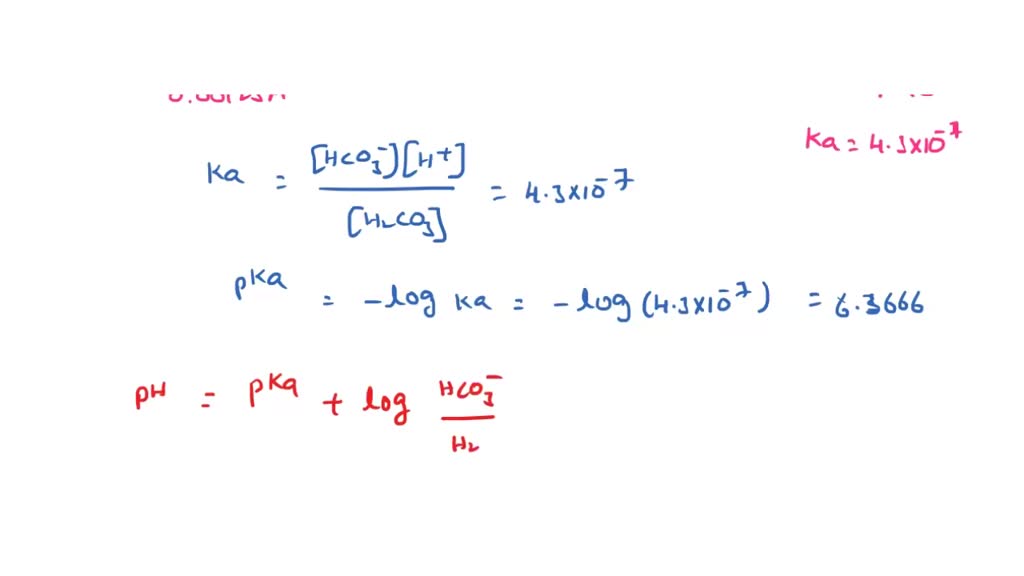
SOLVED: Consider a buffer made of carbonic acid HzCO3 and its conjugate base NaHCO3 with a pH of 6.50. (Ka of H2CO3 is 4.3x10-7) [HCO3 (a) Calculate the log needed to produce