
Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar
![why [Ni(NH3)4]2+ has tetrahedral geometry Explain with vbt - Chemistry - Coordination Compounds - 9638641 | Meritnation.com why [Ni(NH3)4]2+ has tetrahedral geometry Explain with vbt - Chemistry - Coordination Compounds - 9638641 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/ck-files/ck_56065adb7f230.png)
why [Ni(NH3)4]2+ has tetrahedral geometry Explain with vbt - Chemistry - Coordination Compounds - 9638641 | Meritnation.com
![21 15. The oxidation state of Ni and NH, in [Ni(NH3)4]+2 (1) Ni = +2, NH, = 0 [RPMT 2001 (2) Ni = +1, NH, = - 1/6 (3) Ni = +1, NH, = + 1/6 (4) Ni = 0, NH, = +2 21 15. The oxidation state of Ni and NH, in [Ni(NH3)4]+2 (1) Ni = +2, NH, = 0 [RPMT 2001 (2) Ni = +1, NH, = - 1/6 (3) Ni = +1, NH, = + 1/6 (4) Ni = 0, NH, = +2](https://toppr-doubts-media.s3.amazonaws.com/images/4816097/e84878e9-2fe2-45ee-a89d-cd525e6ad65a.jpg)
21 15. The oxidation state of Ni and NH, in [Ni(NH3)4]+2 (1) Ni = +2, NH, = 0 [RPMT 2001 (2) Ni = +1, NH, = - 1/6 (3) Ni = +1, NH, = + 1/6 (4) Ni = 0, NH, = +2
![Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory. Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory.](https://search-static.byjusweb.com/question-images/toppr_ext/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)
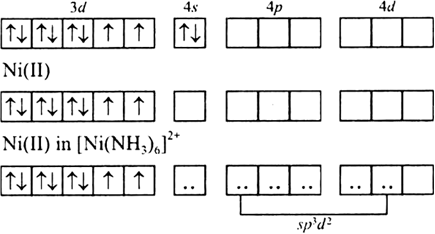

![Answered: [Ni (NH3) 6] C12 Explain the… | bartleby Answered: [Ni (NH3) 6] C12 Explain the… | bartleby](https://content.bartleby.com/qna-images/question/33dc3186-d7b9-4388-8560-215f799e8fe0/013f6e76-4df4-4063-bb5c-5eb17a580f72/z92qakc_thumbnail.png)
![Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook](https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=676374066291248)




![Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table](https://www.researchgate.net/publication/256461217/figure/tbl1/AS:667594596024340@1536178363556/Characteristic-bands-in-infrared-spectrum-of-NiNH3-6-VOO-2-2-NH-3-2.png)