Separation of Ni, Co, and Mn from Spent LiNi0.5Mn0.3Co0.2O2 Cathode Materials by Ammonia Dissolution | ACS Sustainable Chemistry & Engineering

It is an experimental fact that:- DMG+Ni(II) salt+NH_4OH→ Red precipitate Which of the following ... - YouTube

E740: Equilibrium – Complex Ions – Metal + Ammonia Complexes | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

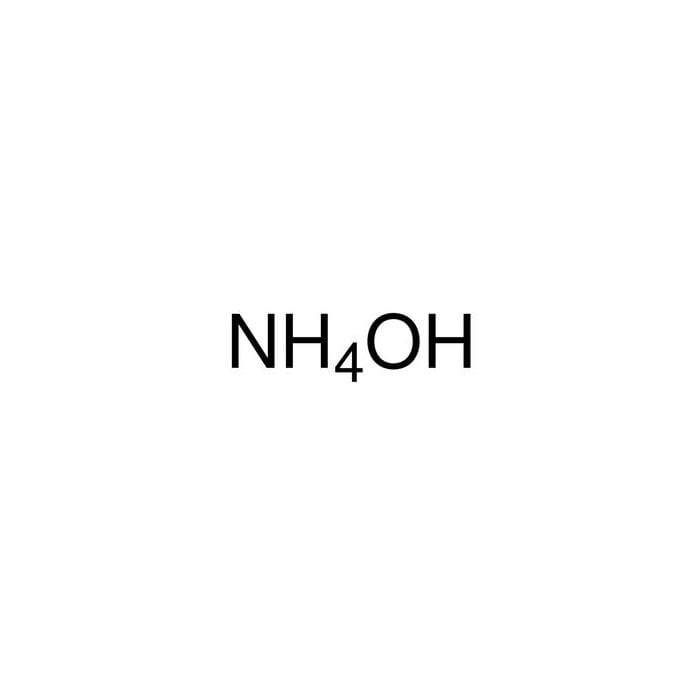
Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Alkaline leaching of nickel bearing ammonium jarosite precipitate using KOH, NaOH and NH4OH in the presence of EDTA and Na2S | Semantic Scholar
27. DMG +NiCl2+NH4OH makes Complex a+ NH4Cl+H2O. What is complex a and find the hybridisation magnetic character and Oxidation state of Nickel in complex a ?

Scanning Electron Microscope image of Ni-BTC MOFs. (a) Ni-BTC Anl ; (b)... | Download Scientific Diagram
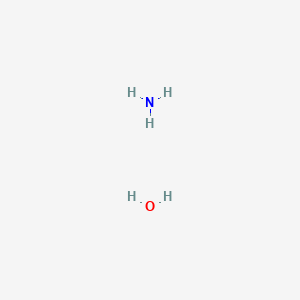
What is meant by aqueous ammonia solution? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
What is nh3 aqueous solution?. NH3 aqueous means aqueous solution of… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Effect of time on Ni and Cd leaching recovery (temperature=45 °C, NH4OH... | Download Scientific Diagram
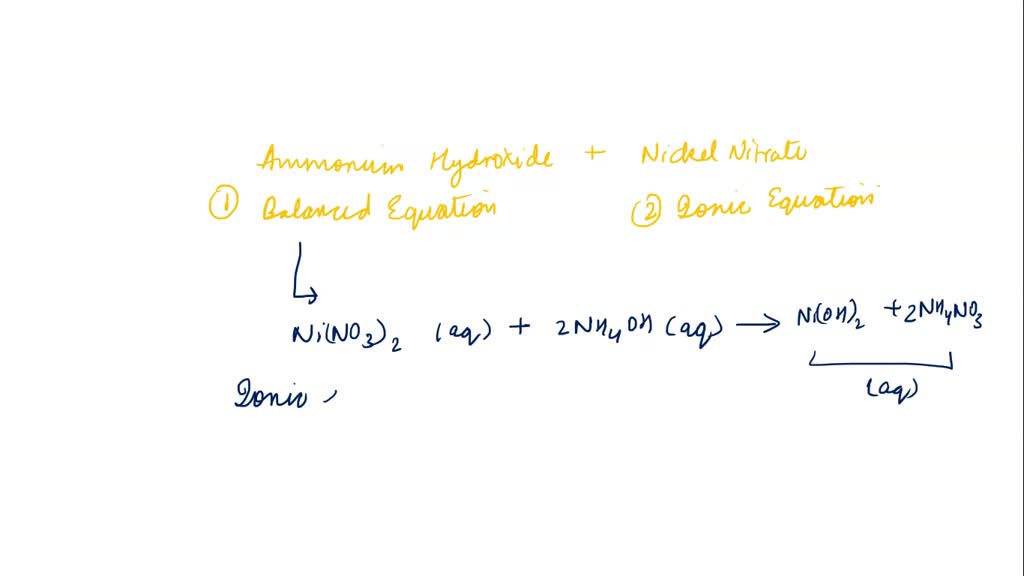
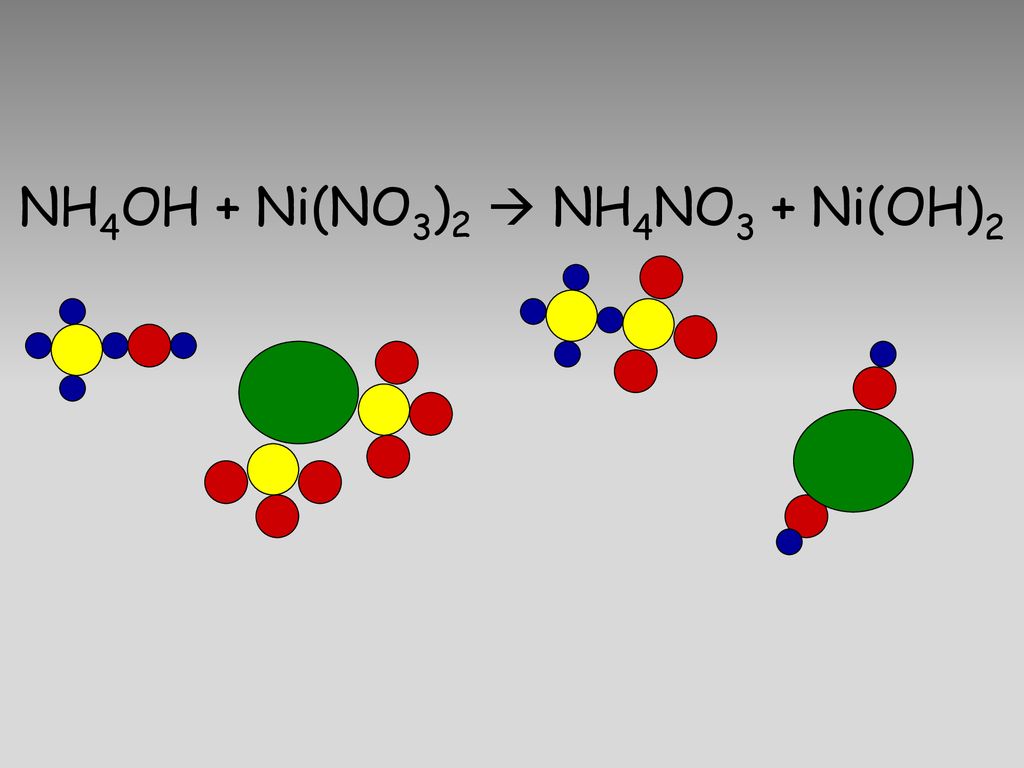
SOLVED: When an aqueous solution of NH4OH is mixed with an aqueous solution of Ni(NO3)2, a pale yellow precipitate forms. Write a balanced molecular equation for this reaction. Write the complete ionic
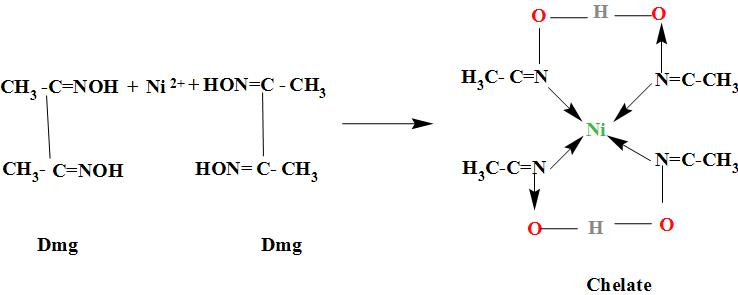
When dimethylglyoxime solution is added to an aqueous solution of nickel (II) chloride followed by ammonium hydroxide, then which of the following statements are incorrect?This question has multiple correct questions(a) No precipitate



/fusion-NH4OH-generation-system-process-flow-diagram.png?width=710&name=fusion-NH4OH-generation-system-process-flow-diagram.png)


/NH4OH%20Fusion%20Chemical%20Blending%20System.jpg?width=1305&name=NH4OH%20Fusion%20Chemical%20Blending%20System.jpg)