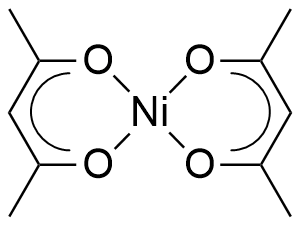
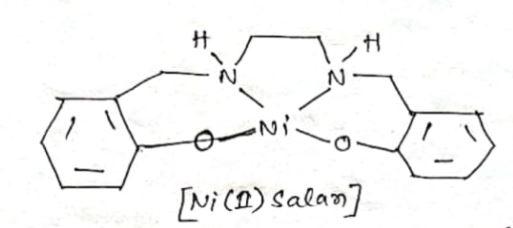
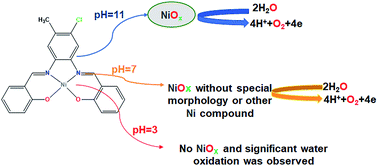
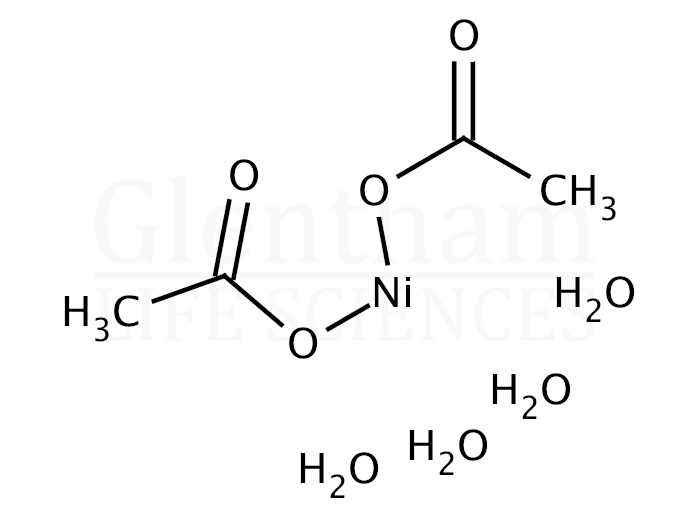
One to Find Them All: A General Route to Ni(I)–Phenolate Species | Journal of the American Chemical Society

Carboxylation of the Ni–Me Bond in an Electron-Rich Unsymmetrical PCN Pincer Nickel Complex | Organometallics

Synthesis, crystal structure, spectroscopic investigations, and computational studies of Ni(II) and Pd(II) complexes with asymmetric tetradentate NOON Schiff base ligand | SpringerLink

Catalysts | Free Full-Text | Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol
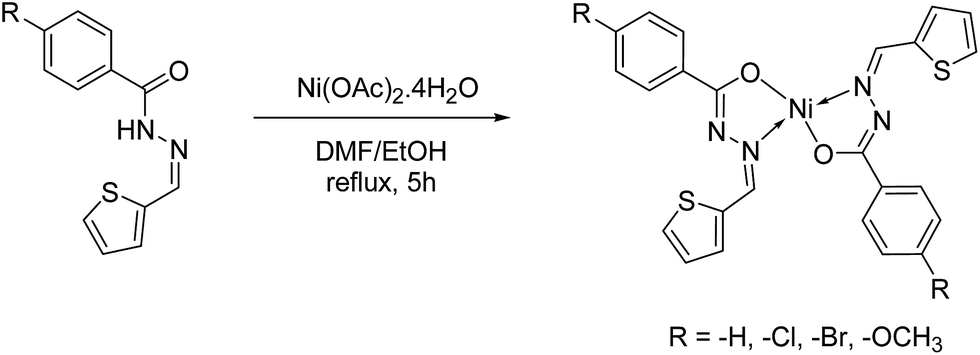
Synthesis, molecular structure and electrochemical properties of nickel( ii ) benzhydrazone complexes: influence of ligand substitution on DNA/protein ... - RSC Advances (RSC Publishing) DOI:10.1039/C5RA19530F
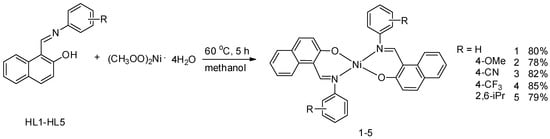
Polymers | Free Full-Text | Mononuclear Nickel(II) Complexes with Schiff Base Ligands: Synthesis, Characterization, and Catalytic Activity in Norbornene Polymerization
![Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones | Nature Communications Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones | Nature Communications](https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fs41467-020-15837-1/MediaObjects/41467_2020_15837_Fig7_HTML.png)
Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones | Nature Communications

Ni‐Catalyzed Regioselective C‐5 Halogenation of 8‐Aminoquinoline and Co‐Catalyzed Chelation Assisted C−H Iodination of Aromatic Sulfonamides with Molecular Iodine - Fernandes - 2022 - Chemistry – An Asian Journal - Wiley Online Library
![Naked d-orbital in a centrochiral Ni(II) complex as a catalyst for asymmetric [3+2] cycloaddition | Nature Communications Naked d-orbital in a centrochiral Ni(II) complex as a catalyst for asymmetric [3+2] cycloaddition | Nature Communications](https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fncomms14875/MediaObjects/41467_2017_Article_BFncomms14875_Fig5_HTML.jpg)
Naked d-orbital in a centrochiral Ni(II) complex as a catalyst for asymmetric [3+2] cycloaddition | Nature Communications

Reactions of Schiff Base‐Substituted Diselenides and ‐tellurides with Ni(II), Pd(II) and Pt(II) Phosphine Complexes - Roca Jungfer - 2020 - European Journal of Inorganic Chemistry - Wiley Online Library
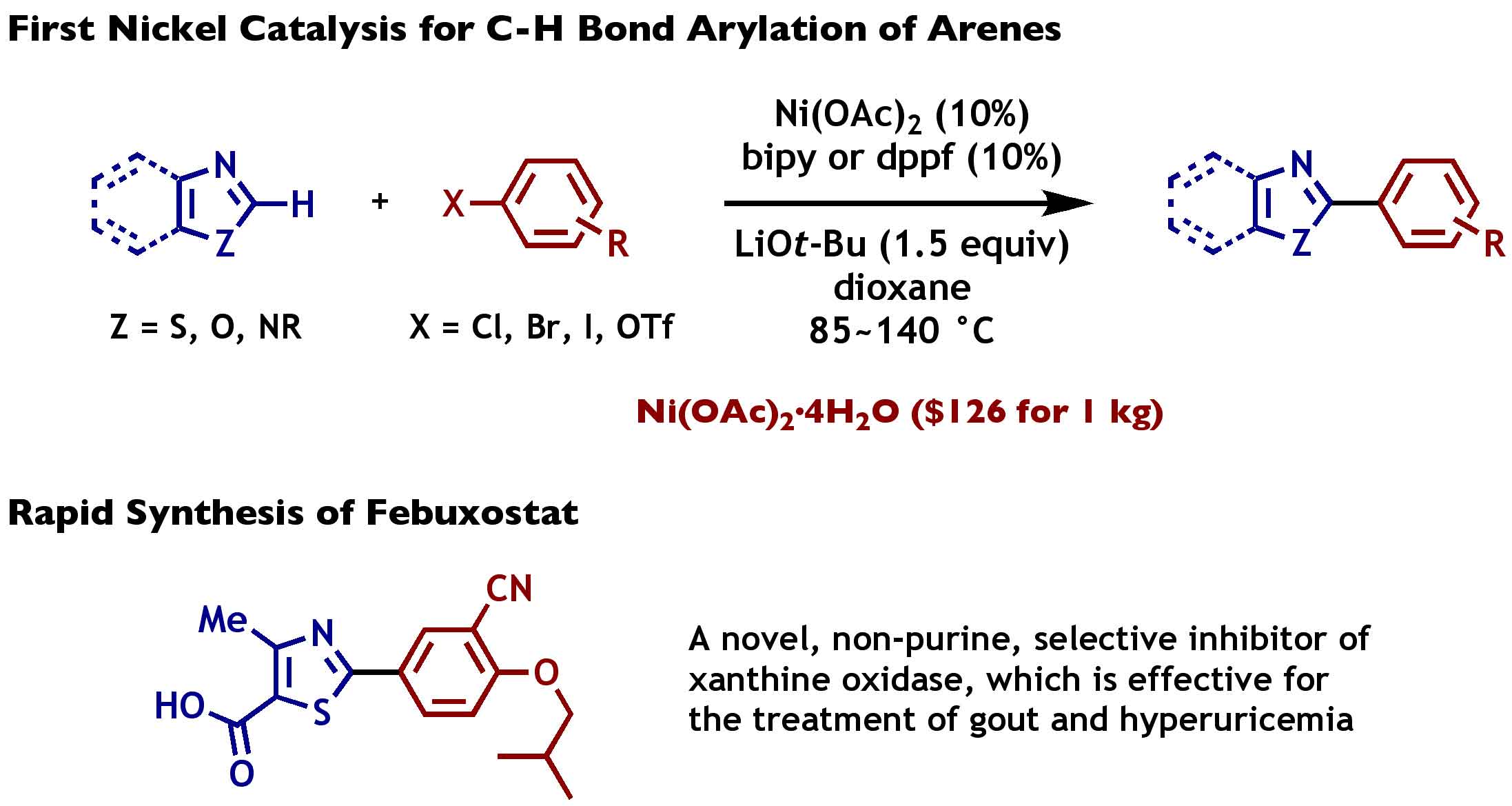
Nickel-Catalyzed Biaryl Coupling of Heteroarenes and Aryl Halides/Triflates | Itami Organic Chemistry Laboratory, Nagoya University
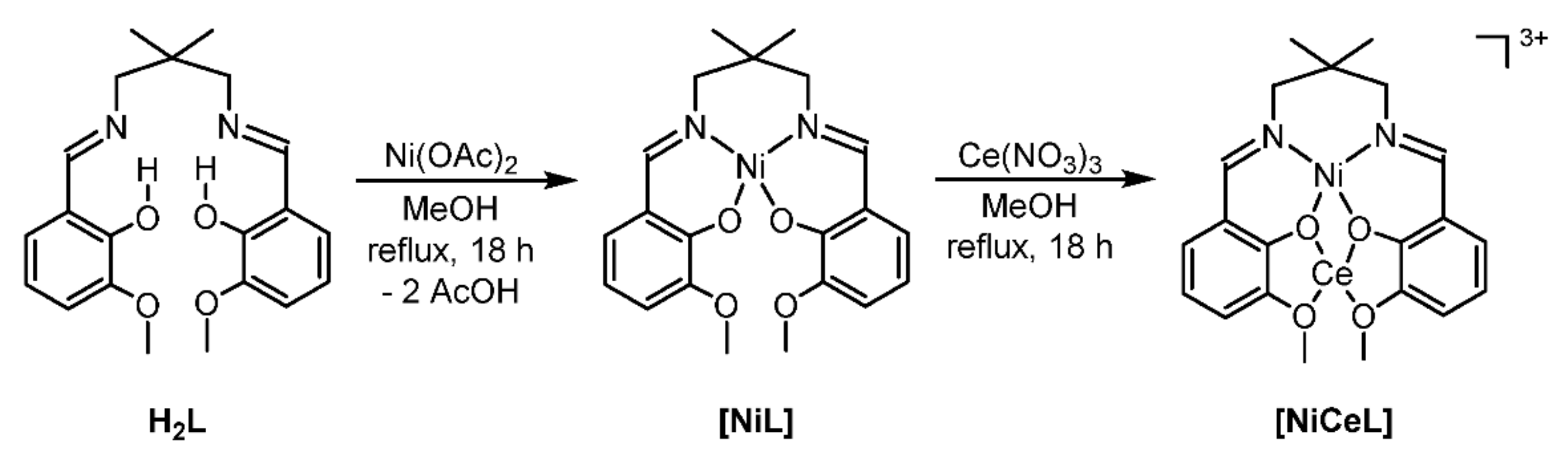
IJMS | Free Full-Text | Mild and Efficient Heterogeneous Hydrogenation of Nitroarenes Facilitated by a Pyrolytically Activated Dinuclear Ni(II)-Ce(III) Diimine Complex

Stereospecific/stereoselective nickel catalyzed reductive cross-coupling: An efficient tool for the synthesis of biological active targeted molecules - ScienceDirect

Ni(II)-Catalyzed Intramolecular C–H/C–H Oxidative Coupling: An Efficient Route to Functionalized Cycloindolones and Indenoindolones | ACS Catalysis

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences